Publications
Table of Contents
A passive flow microreactor for urine creatinine test (2025)
A passive flow microreactor for urine creatinine test (2024)
A Passive Mixing Microfluidic Urinary Albumin Chip for Chronic Kidney Disease Assessment
A Passive Mixing Microfluidic Urinary Albumin Chip for Chronic Kidney Disease Assessment (2018)
Lab-on-a-Chip Platforms for Detection of Cardiovascular Disease and Cancer Biomarkers (2017)
Integrated microfluidic immunoassays for point-of-care diagnostic measurement of human serum cystatin C in chronic kidney disease (2025)
Description
The study demonstrates that these lab-on-a-chip systems can deliver lab-grade analytical performance in decentralized settings, offering fast, reliable, and user-friendly testing at the point of care. By integrating immunoassay technology into portable formats, the research highlights a major step toward accessible, real-time kidney health monitoring — potentially improving early detection and management of chronic kidney disease outside traditional laboratory environments.
Authors: Dumitru Tomsa, Yang Liu, Amir Hossein Abolfathi, Xiaoou Ren, Amanda Stefanson, Nicholas Palmerley, AbdulRazaq A.H. Sokoro, Paul Komenda, Navdeep Tangri, Rene P. Zahedi, Claudio Rigatto, and Francis Lin.
A passive flow microreactor for urine creatinine test (2025)
Description
Researchers have developed a rapid, point-of-care microfluidic device (uCR-Chip) that accurately measures urine creatinine levels within 7 minutes using a simple USB microscope. Designed to work alongside a previously developed urine albumin test, this new chip enables reliable CKD evaluation without lab-based testing. With high precision, a broad detection range, and low matrix interference, the uCR-Chip offers a promising, accessible solution for chronic kidney disease assessment and broader biomarker monitoring.
Authors: Dumitru Tomsa, Yang Liu, Amanda Stefanson, Xiaoou Ren, AbdulRazaq A. H., Sokoro, Paul Komenda, Navdeep Tangri, Rene P. Zahedi, Claudio Rigatto, and Francis Lin
A passive flow microreactor for urine creatinine test (2024)
Description
The uCR-Chip is a low-cost, passive flow microreactor developed to measure urine creatinine levels for point-of-care chronic kidney disease (CKD) screening. Using a USB microscope and colorimetric detection, the chip delivers accurate results within 7 minutes and meets clinical sensitivity standards. Validated against commercial tests, the uCR-Chip offers a reliable and portable solution, especially suited for remote or resource-limited settings, and serves as a foundation for broader disease biomarker detection.
Authors: Dumitru Tomsa, Yang Liu, Amanda Stefanson, Xiaoou Ren, AbdulRazaq A. H. Sokoro, Paul Komenda, Navdeep Tangri, Rene Zahedi, Claudio Rigatto, Francis Lin
A Passive Mixing Microfluidic Urinary Albumin Chip for Chronic Kidney Disease Assessment (2018)
Description
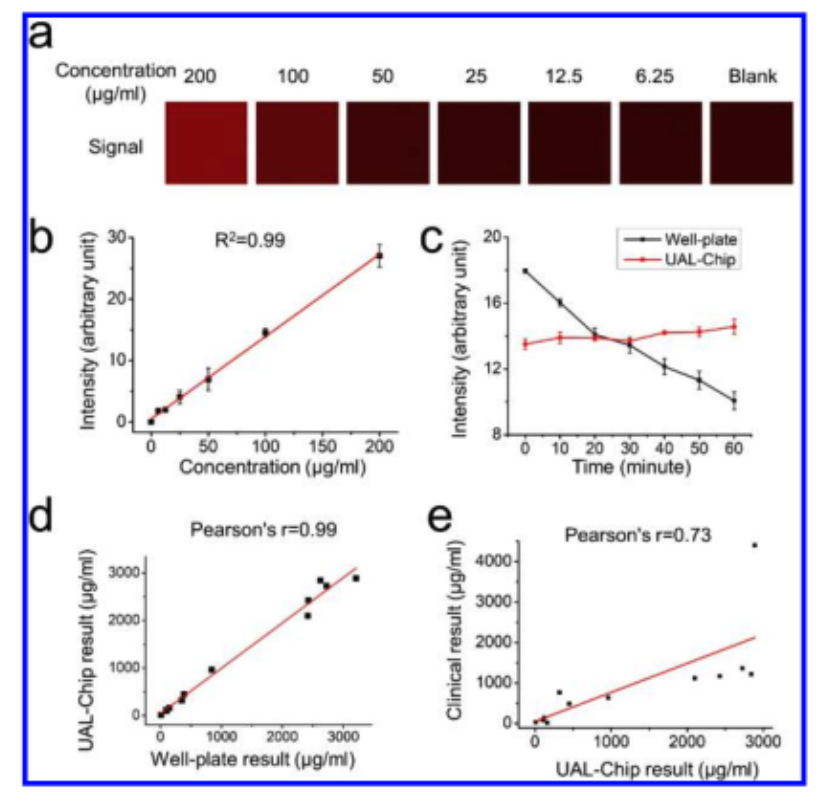
The UAL-Chip is a low-cost, microfluidic device developed for point-of-care detection of urinary albumin, a key biomarker for early-stage chronic kidney disease (CKD). Using a passive mixing system and fluorescent assay, it delivers accurate, stable results within 5 minutes—comparable to lab-based tests but at a fraction of the cost. Validated with CKD patient samples, the UAL-Chip achieved a low detection limit (5.2 μg/mL) and high classification accuracy, making it a practical tool for early CKD screening, especially in remote or resource-limited settings.
Authors: Jiandong Wu, Dumitru Tomsa, Michael Zhang, Paul Komenda, Navdeep Tangri, Claudio Rigatto, and Francis Lin
A Passive Mixing Microfluidic Urinary Albumin Chip for Chronic Kidney Disease Assessment (2018)
Description
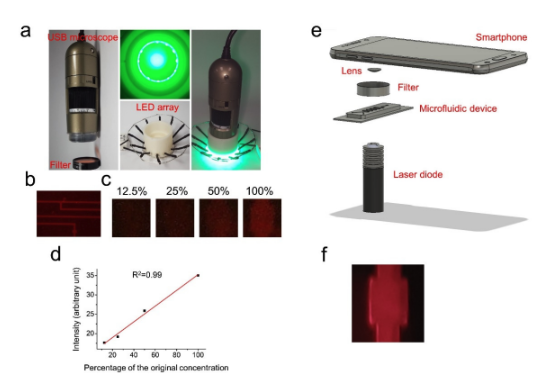
This supplementary study supports the development of the UAL-Chip, a low-cost microfluidic device designed for point-of-care detection of urinary albumin, a key marker for chronic kidney disease (CKD). Clinical validation with 12 patient samples demonstrated strong correlation between UAL-Chip results and clinical gold standards for albuminuria and UACR thresholds. Additionally, two portable imaging prototypes—USB microscope and smartphone-based systems—were tested for feasibility, highlighting future potential for low-resource CKD screening outside traditional lab settings.
Authors: Jiandong Wu, Dumitru Tomsa, Michael Zhang, Paul Komenda, Navdeep Tangri, Claudio Rigatto, and Francis Lin
Lab-on-chip technology for chronic disease diagnosis (2018)
Description
This 2018 npj Digital Medicine review explores the growing role of microfluidics in digital and personalized healthcare. It highlights how microfluidic technologies enable low-cost, miniaturized, and portable diagnostic tools that can be integrated with smartphones and connected platforms. These innovations are paving the way for scalable point-of-care testing, real-time health monitoring, and accessible chronic disease management—especially in remote or resource-limited settings. The paper emphasizes microfluidics as a foundational technology for the future of digital health.
Authors: Jiandong Wu, Meili Dong, Claudio Rigatto, Yong Liu, and Francis Lin
Lab-on-a-Chip Platforms for Detection of Cardiovascular Disease and Cancer Biomarkers (2017)
Description
This 2017 review highlights the advancements in lab-on-a-chip (LOC) technologies for detecting biomarkers related to cardiovascular disease and cancer. It discusses how microfluidic platforms enable rapid, low-cost, and minimally invasive diagnostics using small sample volumes. The paper outlines key innovations in LOC integration, including point-of-care testing, multiplex detection, and real-time monitoring. These developments are accelerating early diagnosis and personalized treatment strategies, especially in resource-limited and decentralized healthcare settings.
Authors: Jiandong Wu, Meili Dong, Susy Santos, Claudio Rigatto, Yong Liu, and Francis Lin
Rapid and Low-Cost CRP Measurement by Integrating a Paper-Based Microfluidic Immunoassay with Smartphone (CRP-Chip) (2017)
Description
This 2017 paper presents a microfluidic CRP-Chip designed for rapid, point-of-care detection of C-reactive protein (CRP), a key inflammation biomarker used in cardiovascular disease and infection assessment. The chip uses passive mixing and fluorescence-based detection to deliver accurate results within 4 minutes, using only a small volume of plasma. Validated with clinical samples, the CRP-Chip shows strong correlation with standard lab tests, offering a cost-effective, portable alternative for decentralized healthcare and timely clinical decision-making.
Authors: Meili Dong, Jiandong Wu, Zimin Ma, Hagit Peretz-Soroka, Michael Zhang, Paul Komenda, Navdeep Tangri, Yong Liu, Claudio Rigatto, and Francis Lin