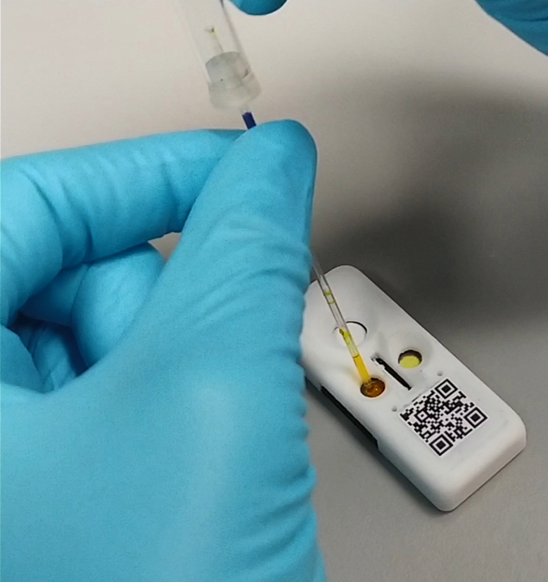
Bring lab-grade CKD testing to your clinic — boost patient care and capture new revenue.
Join our early partners to shape the next generation of kidney care.
Increased Screening = Early Detection
Why the screening gap exists
Despite guidelines from KDIGO, ADA, and the National Kidney Foundation, fewer than 50% of at-risk patients are screened for CKD.
The traditional 4-step process loses patients at every step — resulting in a cumulative completion rate of just 52% even when each step has 85% adherence.
MATLOC eliminates these barriers by enabling testing, results, and action in a single appointment.
What makes MATLOC different?
Lab-Grade Equivalent Results
uACR and Dual-Marker eGFR Testing
Fast, Portable, Affordable
How It Works in 4 Easy Steps
Sample
Insert
Analyze
Act
Clinical Proof and Guidelines Support
“All patients with cardiovascular disease should be screened for kidney disease.”
“In its earlier stages, CKD can be a silent disease … That’s why CKD screening – done using a simple blood and urine test – is so important.”
“Early detection is the best solution to this problem (CKD).”
CKD is a Global Health Issue
Implementation vision
1. Process-mapped to avoid disrupting clinic flow
2. Compatible with EMRs and billing systems
3. Revenue-generating from Day One (post-reimbursement approval)
4. Supporting CMS Value-Based Care objectives
MATLOC offers an early-stage investment opportunity in a disruptive, scalable platform with strong potential for long-term growth.
Invest in the Future of Kidney Care
AssureCKD is positioned at the forefront of a $130B+ market opportunity, transforming the way chronic kidney disease (CKD) is diagnosed and treated. Our flagship product, MATLOC, is designed to revolutionize kidney care by offering affordable, point-of- care screening with lab-equivalent results. We are strengthened by:
Proven Market Opportunities
With CKD prevalence and costs on the rise, MATLOC offers a compelling solution to a significant unmet need.
Validated partnerships
We have established strong relationships with academic institutions such as the University of Manitoba, whose support and research are integral to our product development.
Regulatory & Market Readiness
We are preparing for FDA and Health Canada approval, positioning us to quickly enter both the U.S. and Canadian markets.